PPM to mg/L Converter is a converter used to convert between PPM and mg/L ratios of solution for any substance in water. Additionally, if you specify the molar mass of a substance, our converter will calculate and show you the molarity of the solution. Feel free to enter the values and find out how quickly our converter performs.
In further text we will explain what is PPM, conversion formula and some examples. Meanwhile, check out this category if you need more conversions-related calculators besides our PPM to mg/L Converter. We offer a wide spectrum of other categories, such as math or physics.
Take a look other related calculators, such as:
- Power reducing formula calculator
- Knots to mph
- Mg to ml
- Torr to atm
- Steps to miles
- Grams to tablespoons
PPM – Meaning and Formula
You must have heard about percentages and got familiar with what it represents. Samely, PPM or “parts per million” is a unit of measurement for very small fractions of a substance (one millionth of something). For example, 0.1% of something equals a 1:1000 ratio (one out of a thousand parts). On the other hand, PPM has a ratio of 1:million (one out of a million).
Generally, we choose PPM over other units, when we want to express the presence of a substance in water or soil.
If we use it for water, then 1 PPM is equivalent to 1 mg (milligram) of substance per 1 liter of water. But, on the contrary, if we are measuring the presence of a substance in soil, 1 PPM equals milligram of a substance per kilogram of soil.
Clearly, the PPM unit is only used for a pretty small substance concentration.
PPM = (MS \div MSS) \times million
Where:
MS is mass of the solute and
MSS is mass of the solution.
mg/L – What is?
mg/L or milligrams per liter is another unit of measurement for a small concentration of something. Milligrams per liter represents how much weight of a substance we have per unit of volume in water. For example, 1 mg/L equals 0.001 kg / m3. If we measure the concentration of something in the water, it is always preferred to choose mg/L instead of PPM. PPM and mg/L units are equivalent in many cases, but we can’t say they are equal in all the scenarios.
PPM to mg/L – Conversion
We already mentioned a close connection between PPM and mg/L units of measuring substances concentrations. The point where they part is when we consider the density of a substance. For example, if the density of a substance is high (e.g., oil), then the volume will be small, including the low PPM ratio. In comparison, the volume will increase with the higher PPM ratio when the density is low (e.g., alcohol). Importantly, the mg/L ratio remains the same in both scenarios. Therefore, it would be incorrect to claim that PPM and mg/L unit ratios remain the same.
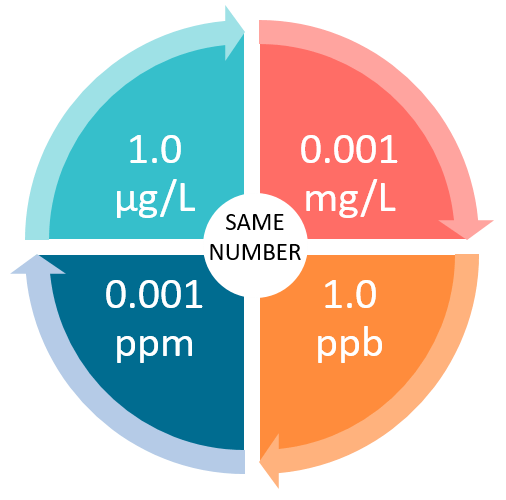
Let’s see how the conversion works:
- First, choose a solute that you want to use in the conversion. For example, we will pick a solute with a density of 800 kg/m³. Our substance will be diluted in the water.
- Secondly, we need to find the PPM ratio. Let’s assume for this solution, the PPM ratio is 1400 PPM.
- Apply PPM to mg/L converter formula and calculate it –
Which means that, 1400 PPM equals 1120 mg/L of our substance in the water.
Note: If our substance that we dissolve in the water has the density close or almost identical to the density of water (1000 kg/m³), in that case, 1 PPM is equivalent to 1 mg/L.
Molar Concentration Formula
Suppose we use the previous formula to calculate a solution’s mg/L (milligramliter). In that case, we can expand it, and just by adding one more property – we can calculate the molar concentration of a substance. It represents how many moles are within a liter of the solution.
To calculate molarity, first, we need to know the mass (g/mol) of the substance that you dissolve in the water.

M = mg/l \div (SMM \times 1000)
Where:
M is Molarity
SMM is the substance’s mass
Molarity to PPM – Conversion
Suppose we have the molarity of 0.5 M, and we would like to convert molarity to PPM. How do you accomplish that?
First step: Take the molarity and multiply it by the substance’s molar mass. We will use the mass of oil (900 g/mol).
0.5 \times 900 = 450 g/L
Second step: Multiply it by 1000 to convert grams to milligrams.
450 \times 1000 = 450,000 mg/L (PPM of the substance in the water)
mg/L to PPM Conversion Table
Here is the table of some possible conversions from mg/L to PPM:
| Milligram/liter [mg/L] | Part/million [ppm] |
| 0.01 mg/L | 0.010011423 part per million (ppm) |
| 0.1 mg/L | 0.1001142303 part per million (ppm) |
| 1 mg/L | 1.001142303 part per million (ppm) |
| 2 mg/L | 2.002284606 part per million (ppm) |
| 3 mg/L | 3.003426909 part per million (ppm) |
| 5 mg/L | 5.005711515 part per million (ppm) |
| 10 mg/L | 10.01142303 part per million (ppm) |
| 20 mg/L | 20.02284606 part per million (ppm) |
| 50 mg/L | 50.05711515 part per million (ppm) |
| 100 mg/L | 100.1142303 part per million (ppm) |
| 1000 mg/L | 1001.142303 part per million (ppm) |
PPM to mg/L Converter – How to Use?
This section will walk you through the step-by-step process of using our PPM to mg/L Converter.
Follow the steps below:
- Determine the density of your solution (I chose 700 kg/m³)
- Find the PPM ratio (Suppose you have the solution with 1120 PPM)
- Enter those values in the calculator. It will give you milligrams per liter (784 mg/L)
- 1120 PPM is equivalent to 784 mg/L of our substance in the water
PPM to mg/L Converter – Example
We have tackled many topics and formulas in this article, so the best way to wrap things up is to end with an example. In this example, you will see how to use the density of a substance, convert PPM to mg/L and calculate the molarity using the mass (g/mol) of the particular substance. Let’s get started!
Scenario: Suppose you have a substance of solute density 700 kg/m³, and you find that its PPM ratio is 1414 PPM. How do you convert it to mg/L using our calculator, and how do you calculate the molar concentration if the mass is 25 g/mol?
You can solve the conversion from PPM to mg/L using the formula below:
mg/L =PPM \times Density \div 1000 mg/L = 1414 \times 700 \div 1000 = 989.8Therefore, 1414 PPM equals 989.8 mg/L with a density of 700kg/m³.
We have finished the first part of the task, now let’s calculate the molarity.
All you need to do is, enter the mass (g/mol) of your substance, and our converter will automatically give you the molarity, which is 0.03959 M in our case.
FAQ
What is mg/L?
mg/L is a unit of measurement that represents how much weight of a substance is found per unit of volume in water.
Convert 100 PPM to mg/L?
If we talk about water, 100 PPM when converted into milligrams per liter equals 100.1142303 part/million (ppm). However, this is not the case if your substance is diluted in something other than water.
How do you calculate PPM in water?
If we calculate the PPM ratio in pure water at the standard pressure and temperature, we generally assume that 1 PPM is equivalent to 1 mg/L.
Is PPM the same as mg/L?
PPM is only considered equal to mg/L if we dilute our substance in the water. However, if we dilute it in other bases with different densities, PPM is not equivalent to mg/L.
What is the molar mass of water?
If we look at the structure of water, we see that one mole of water consists of two moles of hydrogen atoms and one mole of oxygen atoms. Therefore, one mole of hydrogen equals 1 g/mol, whereas one mole of oxygen equals 16 g/mol. As a result, we have this equation below:
Water = (2 \times 1) + (1 \times 16) = 18 g/mol
More precisely, it’s 18.01528 g/mol.
What is the molar mass of salt?
One mole of NaCl consists of one sodium atom and one chlorine atom.
The molar mass of a sodium atom is 22.989770 g/mol, while for a chlorine atom is 35.453 g/mol.
If we add the masses, you will get the total g/mol mass of salt:
Salt = 22.989770 + 35.453 = 58.44277 g/mol.
