The article discusses assessing the anion gap based on the calculated sodium, chloride, and bicarbonate levels in the blood serum. You can do all this with our calculator, which offers you an advanced way of working with the option of entering potassium levels to obtain the most accurate results. We will explain the meaning of the anion gap, its calculation, and the way the calculator works through the title sections.
Take a look other related calculators, such as:
- Mentzer index calculator
- Glycemic load calculator
- Micronutrient calculator
- Venous blood gas interpretation calculator
What is the anion gap?
To better understand how the calculator works, we need to introduce you to the essential information. To begin with, we will say something more about the anion gap itself. This value indicates the difference between positive ions – cations and negative ions – anions in the blood. It is calculated based on electrolyte panels or the type of blood test. In this process, the concentration of anions and cations is monitored, and specific chemical elements in the blood such as chloride, bicarbonate, sodium, and potassium are. The concentration of potassium is in a smaller amount compared to the other mentioned elements, so in practice, it is not used to calculate the anion gap. To maintain neutrality in the body, the total charge of the cation must be equal to the amount of the anion in the blood. The anion gap is the best way to show a clear picture of unmeasured anions and cations because many blood tests cannot measure all types of ions. When we talk about the practical application of this method, the calculation of the anion gap is very often used to identify acid-base disorders in a situation where the concentration of acids and bases is imbalanced.
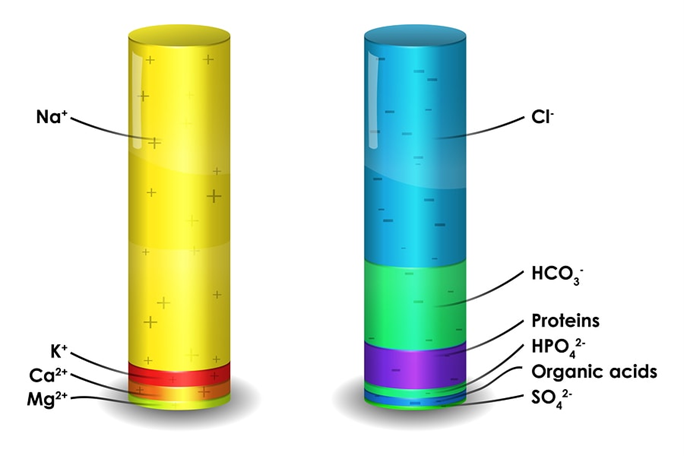
How to calculate the anion gap?
To better understand the equations for calculating the anion gap, we will assume a situation that can occur in practice. The assumption is that it is a lady in her 50s who has been diagnosed with severe acute diarrhea that has been going on for several days. The values of blood elements performed according to laboratory tests are:
Na+, 156 mEq/L
K+, 3.2 mEq/L
Cl–, 132 mEq/L
HCO3–, 16 mEq/L
At the moment, our calculator has a huge role to play in making calculations easier. All you have to do is enter the results in the fields provided for each of the elements, and the calculator will do its job in the background. According to the formula, the anion gap in this case will be:
AG = [Na+] – ([Cl–] + [HCO3–]) = 156 – (132 + 16) = 8
or
AG = AG = [Na+] + [K+] – ([Cl–] + [HCO3–]) = 156 + 3.2 – (132 + 16) = 11.2 (including potassium level)
According to the obtained results, the value of the anion gap is within the average level and is 8 mEq/L or 11.2 mEq/L. The recommendation for treatment in this patient’s case would be to increase fluid and electrolyte intake to treat diarrhea.
Anion gap calculation formula
The formula for calculating the anion gap contains data on the level of electrolytes in the blood serum. In everyday use, a formula is used that looks like this:
AG = [Na+] – ([Cl–] + [HCO3–])
Na+: sodium cations
Cl–: chloride anions
HCO3–: bicarbonate anions
In addition to this formula, the one that includes the percentage of potassium concentration is in use, which in many cases is very low, but the result is much more accurate if you include the value of potassium. The equation has this appearance:
([Na +] + [K +] + [UC]) = [[Cl–] + [HCO3–] + [UA])
Rearrangement shows:
([Na+] + [K +]) – [[Cl–] + [HCO3–]) = [UA] – [UC]
Anion Gap = UA – UC
UA: number of unmeasured anions
UC: number of unmeasured cations
What it looks like in the end:
AG = [Na+] + [K+] – ([Cl-] + [HCO3–])
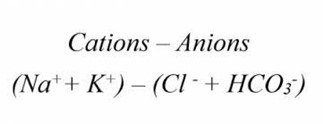
What is a normal anion gap?
The normal level of anion gap values may vary depending on the equipment and techniques used in specific laboratories. If the results you get are outside the normal range, it does not necessarily mean a problem. Also, a value within the normal level does not necessarily indicate the absence of a specific health condition. It is always necessary to contact the qualified medical staff for a more detailed definition and reading of the results obtained after testing. The values obtained after the anion gap test are expressed in milliequivalents per liter (mEq/L). Values marked as normal are those between 3 and 11 mEq/L. If the value of potassium levels is included in calculating the anion gap, this limit is between 5 and 16 mEq/L.
If your results vary and deviate from the limits of the normal level, then it can be a sign that you have some problems in the body. Suppose the results show a high anion gap (> 11 mEq/L or> 16 mEq/L including potassium). In that case, it is possible that you have high anion gap acidosis or that the level of acids in your body in the blood serum is higher than normal. Acidosis (ketoacidosis or lactic acidosis) can be caused by dehydration, diarrhea, or strenuous exercise and can also indicate some more serious problems such as diabetes or potential kidney disease.
On the other hand, if your results showed a low anion gap, it may indicate a low level of albumin, more precisely, protein in the blood. Low anion gap refers to values <3 mEq/L or <5 mEq/L including potassium levels. This condition of recording lower albumin protein levels than normal levels is called hypoalbuminemia. It can also signify that there are certain problems with the kidneys, heart, or cancer. These cases are not unknown in practice, and in these cases, tests are often repeated to ensure the accuracy of the measured values.
How to calculate anion gap without HCO3?
The delta gap shows the phenomenon in which certain changes may occur in terms of bicarbonate levels.
Delta gap = (change of anion gap) – (change of bicarbonate)
In this situation, it is assumed that the level of normal anion gap is 12, and the level of normal HCO3 is 24. In addition to the mentioned equation, a simplified equation is used, which does not take into account the value of bicarbonate (HCO3):
Delta gap = (Na+) – (Cl–) – 36
A delta gap is a tool that shows whether or not there is normal metabolic acidosis of the anion gap. If the recorded value is zero, it represents the expected value of the delta gap. If the level of bicarbonate changes to a lower extent than the anion gap, this will indicate the presence of alkalosis in the body. Otherwise, if the change in bicarbonate is far more significant concerning the difference in the anion gap, it shows the existence of acidosis. Because of all the above, a bicarbonate value is not required for the calculation because it is an advantage in obtaining more accurate information.
How to calculate the anion gap in DKA?
We distinguish two states of a metabolic acidosis of the anion gap in medical practice: diabetic ketoacidosis and salicylate poisoning. When the disease is called diabetic ketoacidosis, the patient has symptoms such as vomiting, abdominal pain, increased urination, and in some cases, the appearance of a fruity odor in the breath. Patients diagnosed with diabetic ketoacidosis in most cases have a state of hyperglycemia. In these cases, it is not necessary to adjust the sodium level in the blood serum to calculate the anion gap because this will affect the dilution of chloride and bicarbonate in the blood, resulting in an erroneously read high anion gap. Diabetic ketoacidosis is recognizable by a bicarbonate level <18 mEq/L and hyperglycemia above 250 mg/dL. Regarding the definition of the anion gap in the case of DKA, if the value is greater than 10 mEq/L, then it is a mild DKA, and if the value is greater than 12 mEq/L, then it is a more severe form.
Why is the anion gap corrected for albumin?
If low albumin protein levels occur in critically ill patients, it is necessary to adjust the obtained value to variations in albumin concentration in the blood serum. In case of detection of hypoalbuminemia, the value of the anion gap should be increased by 2.3 to 2.5 mEq/L for each recorded decrease in blood albumin of 1 g / dL. Many reasons can lead to the appearance of this medical condition, like bleeding, intestinal obstruction, and cirrhosis of the liver. Decreased albumin may also mask a slight increase in the anion gap. That is why the correction is made using the with Figge-Jabor-Kazda-Fencl equation shown below:
Albumin-Corrected Anion Gap = Anion Gap + 2.5 x ([Normal Albumin] – [Measured Albumin])
