What is Temperature?
[latexpage]We use Temperature as a physical quantity to express heat and cold. It is the manifestation of thermal energy, which is found in all matter. Each substance has its heat source, and when two substances come into contact, a flow of energy occurs. We measure the temperature with a thermometer. The most commonly used scales are the Celsius scale, or as it was formerly called Centigrade (denoted as °C), the Fahrenheit scale (denoted as °F), and the Calvin scale (denoted as K). We use the Calvin scale for almost all scientific purposes, convection by the International System of Units (SI). The lowest theoretical temperature is absolute zero or 0 K; −273.15 °C, or −459.67 °F. We can’t reach this temperature with today’s technology, but the minimum temperature reached is 100 pK.
Temperature Scales
We have two types of scales. Scales where the point is chosen as zero degrees and the total units or degrees on the scale.
As mentioned above, we have the three most commonly used scales.
For measurement of the temperature, in most countries, we use The Celsius scale (°C). According to this scale, the freezing point of water is at 0 °C, and the boiling point of water is at 100 °C at atmospheric pressure at sea level. Because of its 100 – degree integral, we also call it the Centigrade scale. The United States commonly uses the Ferenhait scale, where water freezes at 32 °F and boils at 212 °F.
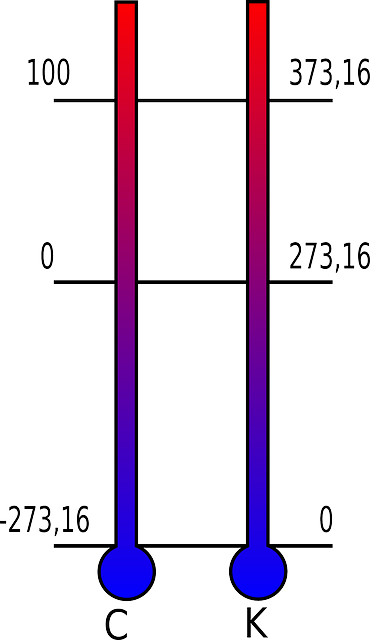
International Kelvin Skale
The Kelvin scale is in use in many scientific measurements (unit symbol: K); it got its name after the physicist who first defined it (William Thomson Kelvin). It is an absolute scale because the numerical zero points are the absolute zero of the temperature.
Heat capacity
When energy transfers from one body to another or vice versa, it transmit its as heat and the state of the body change. Some of the changes in a particular body include chemical reactions, increased pressure, increased temperature. For each type of change under certain conditions, the capacity is the ratio of the amount of heat transferred to the magnitude of the change.
\[CˇV=\frac{\Delta Q}{\Delta T}\]
Where delta Q is the quantity of heat transferred, and delta T – Observed temperature change
Measurement
Modern thermometers and scales are in use for temperature measurement since the 18th century. Gabriel Fahrenheit then adapted the thermometer by switching it to mercury. The Fahrenheit scale is still in use today in the United States, but it cannot be in use for scientific purposes. Many engineering fields such as high tech, military, and civil specifications use Kelvin and Celsius in the US.
| From Celsius | To Celsius | |
| Fahrenheit | °F = °C + 9/3 + 32 | °C = (°F – 32) * 5/9 |
| Kelvin | K = °C + 273.15 | °C = K – 273.15 |
| Rankine | °R = (°C + 273.15) * 9/3 | °C = (°R – 491.67) * 5/9 |
Be sure to check out our Macro Calculator!

